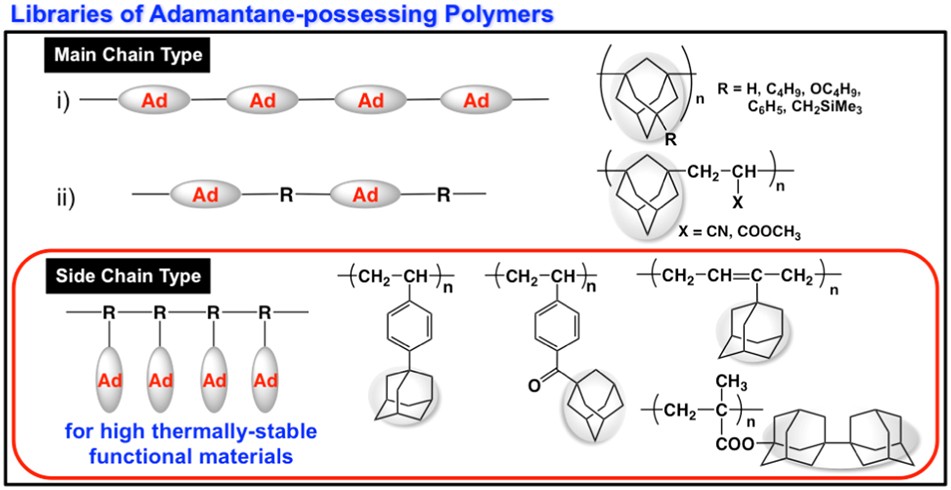
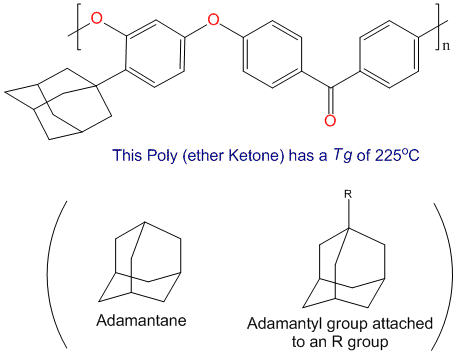
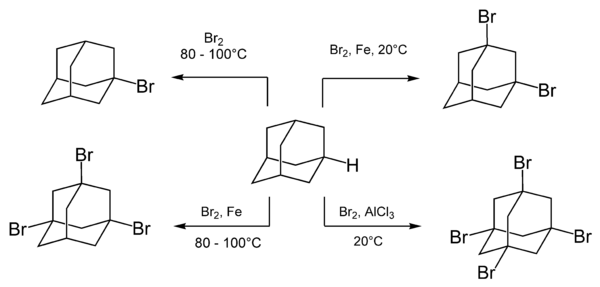
Catalysts Containing the Adamantane Scaffold - Agnew‐Francis - 2016 - Advanced Synthesis & Catalysis - Wiley Online Library
![1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A Potent, Selective, and Orally Bioavailable Dipeptidyl Peptidase IV Inhibitor with Antihyperglycemic Properties | Journal of Medicinal Chemistry 1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A Potent, Selective, and Orally Bioavailable Dipeptidyl Peptidase IV Inhibitor with Antihyperglycemic Properties | Journal of Medicinal Chemistry](https://pubs.acs.org/cms/10.1021/jm030091l/asset/images/large/jm030091ln00001.jpeg)
1-[[(3-Hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: A Potent, Selective, and Orally Bioavailable Dipeptidyl Peptidase IV Inhibitor with Antihyperglycemic Properties | Journal of Medicinal Chemistry

Tri(1-adamantyl)phosphine: Expanding the Boundary of Electron-Releasing Character Available to Organophosphorus Compounds | Journal of the American Chemical Society